Managing ABS at a University Museum

-
Region
Global -
Country
USA -
Topic
Policy and Advocacy -
Type
Case Study
Developing an Institutional Policy and Tools for ABS
Example provided by: Breda M. Zimkus, Cryogenics Collections Manager for Genetic Resources, Museum of Comparative Zoology, Harvard University
The Museum of Comparative Zoology (MCZ) is a private center for research and education focused on the comparative relationships of animal life. The MCZ was founded in 1859 by an act, signed into law, of the Commonwealth of Massachusetts opened its doors the same year. The MCZ collections are comprised of approximately 21-million extant and fossil invertebrate and vertebrate specimens, which continue to be a focus of research and teaching for MCZ, Harvard University and outside students and researchers.
Development of ABS Policy
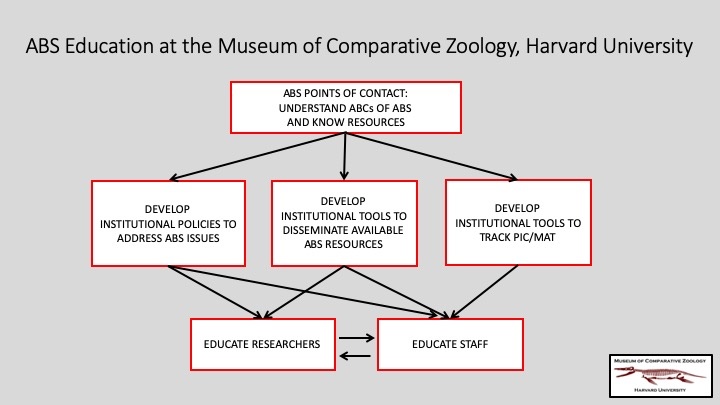
MCZ’s Director of Collections Operations, who supervises all museum collections, and the Cryogenics Collections Manager for Genetic Resources have been designated as the MCZ’s points of contact regarding ABS, understanding the so-called ABCs of ABS and keeping up-to-date with available resources so that they may assist staff and researchers (whether from Harvard or elsewhere). Together they are:
- Developing institutional policies to address ABS issues
- Developing institutional tools to disseminate available ABS resources
- Developing institutional tools to track Prior Informed Consent (PIC) and Mutually Agreed Terms (MAT)
It is the responsibility of the relevant MCZ researcher to obtain permission in the form of PIC and establish MAT to share any benefits arising from the utilization of genetic resources before collecting specimens/samples in foreign countries. Museum guidelines regarding obtaining PIC and establishing MAT are currently being written, and they will be provided to all researchers conducting international fieldwork. Upon return, researchers must provide all documentation (e.g., permits, agreements, certificates) to the relevant museum department with the specimens for the accessioning process.
Awareness-raising and information-sharing
The ABS Points of Contact have made a number of efforts to educate both MCZ staff and researchers regarding how the Nagoya Protocol and ABS will change current practices both in the museum and in the field. The education of staff regarding the Nagoya Protocol and ABS has generally been done during regular meetings and includes discussion about the types of documentation that must be obtained with all accessions (see Data Management for more information). Researchers (e.g., curators, postdoctoral fellows, graduate students based at MCZ and other Harvard University bodies) have been provided with information regarding scientific permitting best practices before they collect specimens/samples, and they have been directed to the ABS Clearing-House for country-specific information.
Data management
The museum has migrated its legacy specimen records from multiple independent sources to a single centralized database, MCZbase, a unique instance of the Arctos platform. This new database conforms to recognized standards for natural history collections including the ability to manage and track collection management duties, as well as make the MCZ’s historically and scientifically significant holdings available to researchers and the public alike. In addition, various forms of digital media, NCBI accessions (e.g., GenBank), and other information are linked to the relevant specimen record(s) and are readily accessible through the standard searching protocols.
The museum has made a number of changes to the database to improve digital access to permits and other legal documentation. Permits and other legal documentation (e.g., Prior Informed Consent, Mutually Agreed Terms, Internationally Recognized Certificate of Compliance, import permit, export permit) can be tracked in the database using specific core information, categorized into permit type, and uploaded for easy viewing. Each acquisition is given an accession number, which is a unique identifier associated with all the specimens or samples associated with it, and permits can be linked to accessions for transparency and trackability. The addition of specimens to a loan in MCZbase also adds all relevant documentation related to each accession included in the loan, so staff may view any pertinent requirements or restrictions associated with the specimens or samples before sending. The museum is currently in the testing phase for these recent database changes and will continue to make improvements with staff and researcher feedback.
Wider engagement
MCZ’s Director of Collections Operations and Cryogenics Collections Manager for Genetic Resources recently hosted a workshop to address legal concerns associated with digitized collections and mitigating these issues using digital means. Participants with practical knowledge of how biological collections should manage legal issues in regards to changing policy, utilizing cyberinfrastructure, and advising stakeholders used compliance with the Nagoya Protocol as a test case to investigate how U.S. institutions must respond to the need for increased transparency of their biodiversity collections and the required digital tracking. For a webinar that summarizes the discussions and findings, see the Biodiversity Collections Network website.
The natural history community is aware that Access and Benefit-Sharing (ABS) poses a number challenges for museums, but we believe that this provides and opportunity to prioritize developing policies and procedures associated with tracking compliance documentation. Staff from the MCZ are members of the Society for the Preservation of Natural History Collections (SPNHC) Best Practices Committee, and Global Genome Biodiversity Network (GGBN) Policy Task Force. Both groups are working to develop best practices and tools to increase understanding about ABS.


